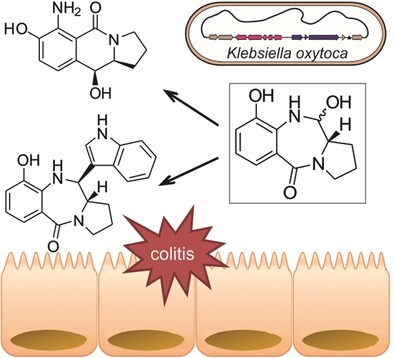
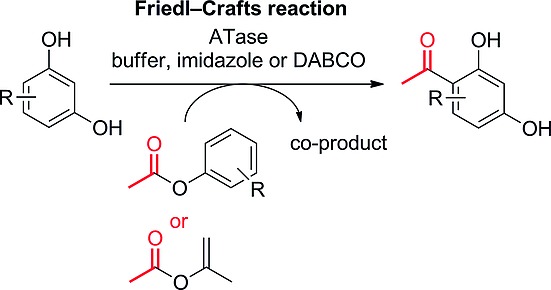
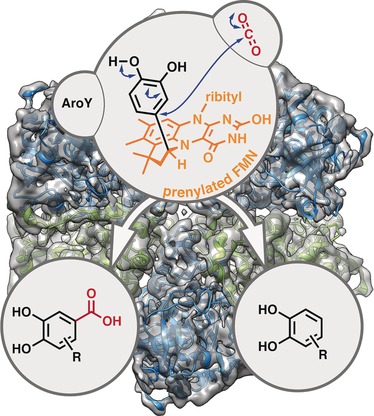
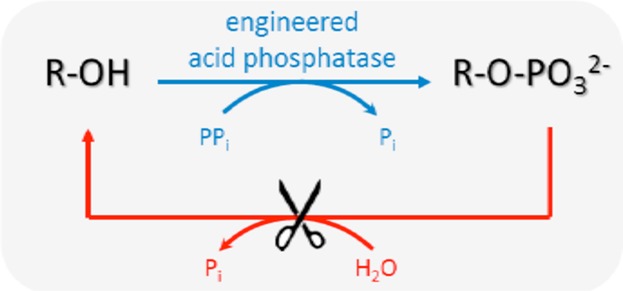
Paper
Coupling photocatalytic trifluoromethylation with biocatalytic stereoselective ketone reduction in continuous flow
A. Valotta, J. Maderbacher, T. Reiter, W. Krouti, H. Gruber‑Woelfler
Chem.Pap. 2024, 78, 7973-7986
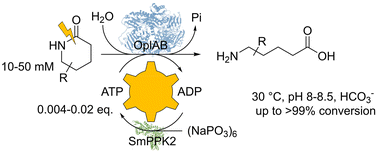
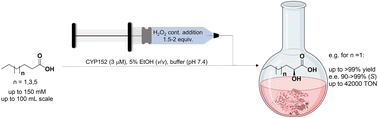
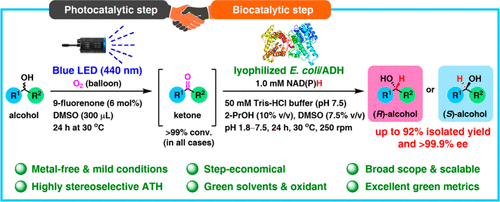
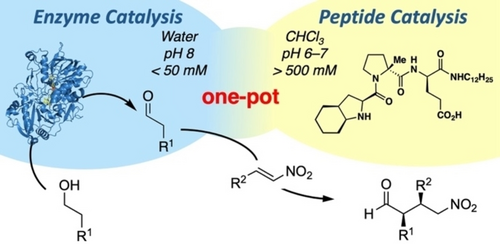
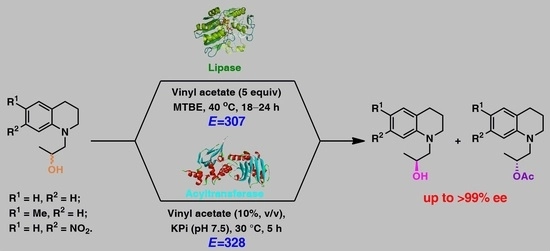
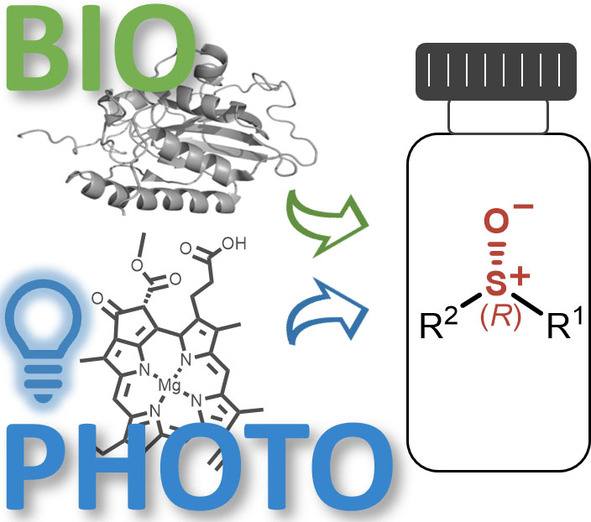
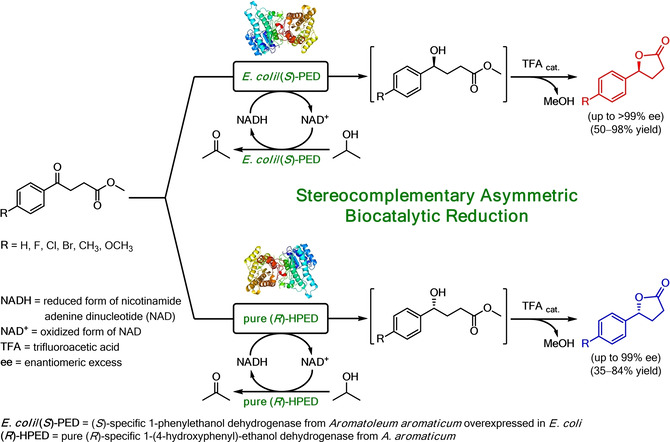
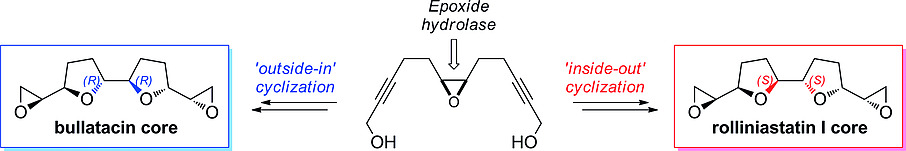
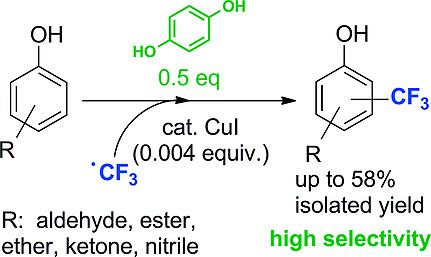
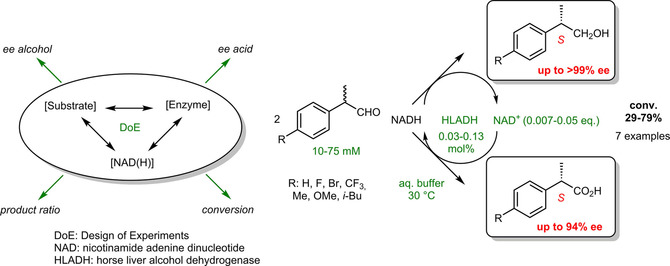
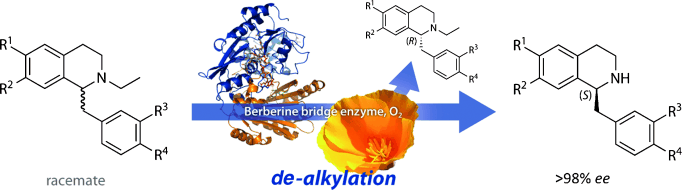
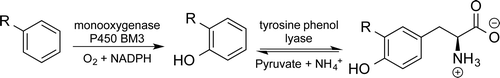
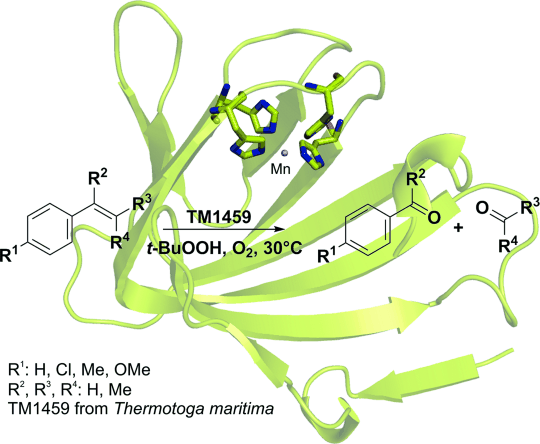
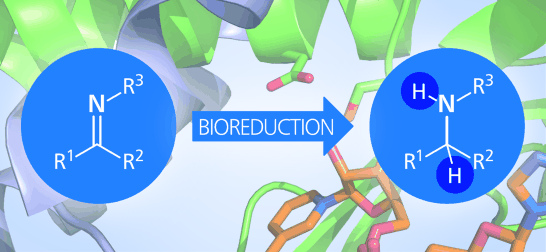
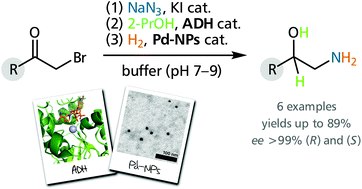
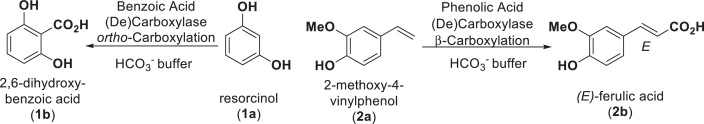
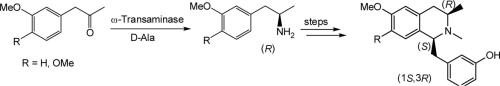
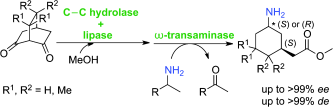
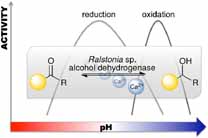
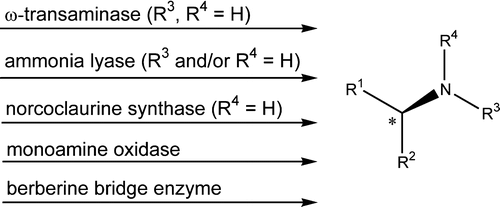
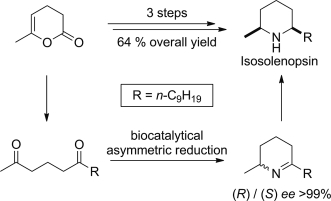
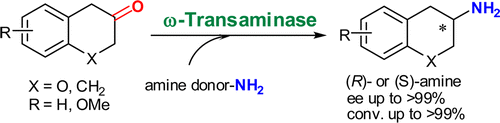
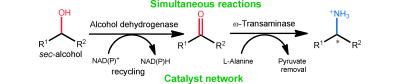
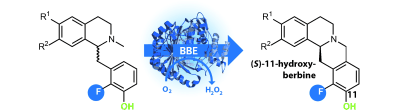
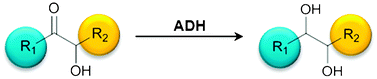
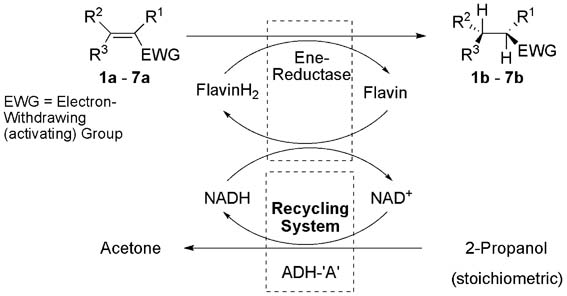
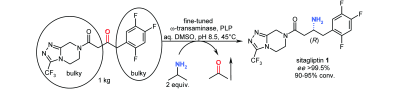
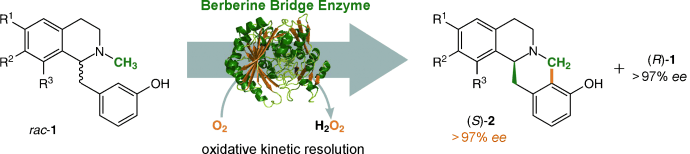
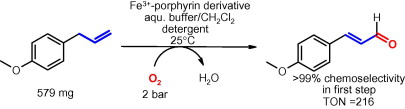
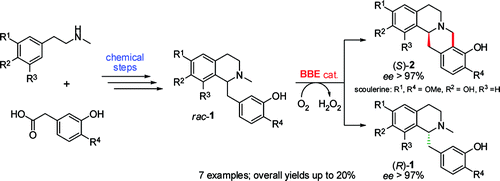
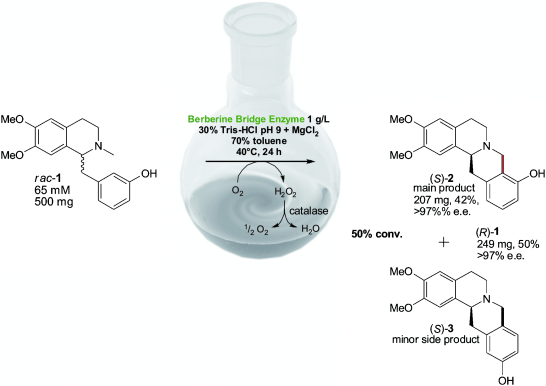
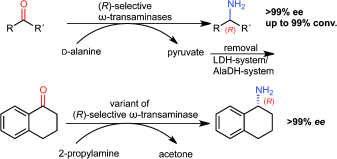
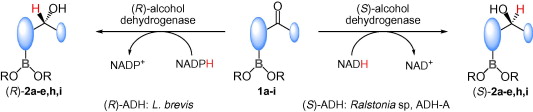
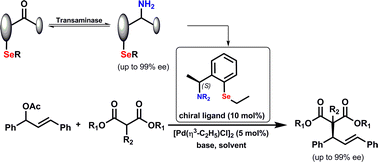
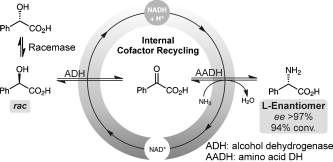
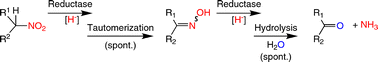
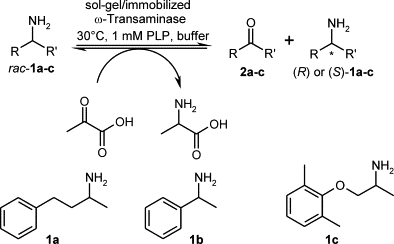
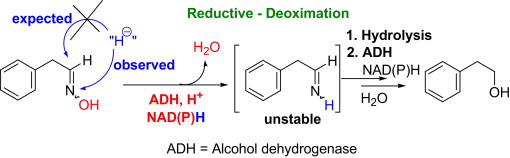
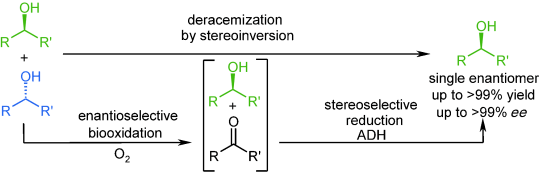
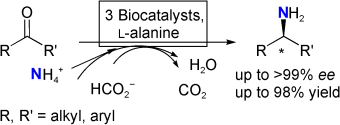
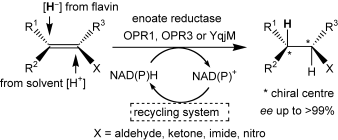
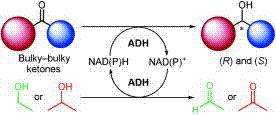
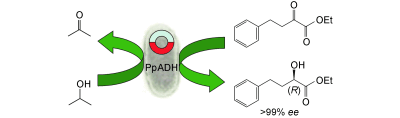
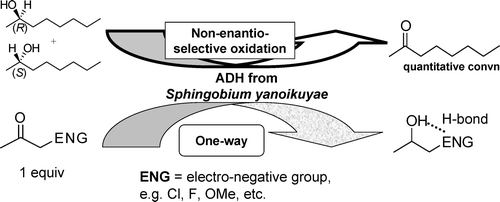
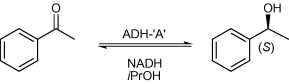
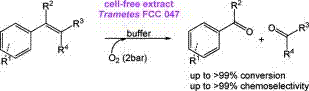
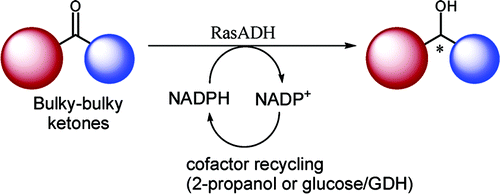
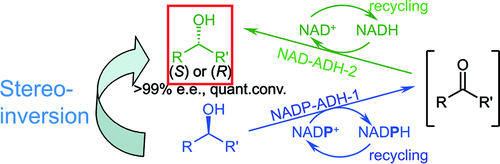
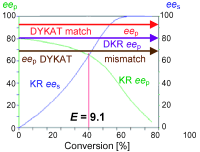
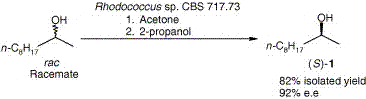
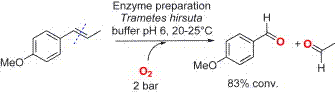
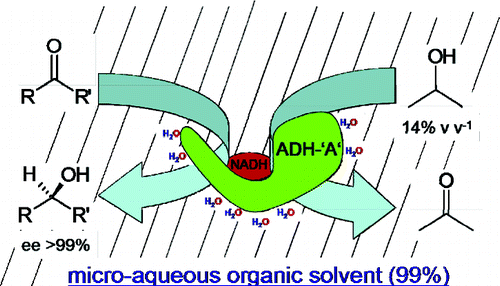
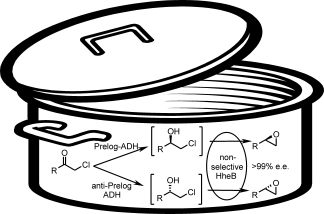
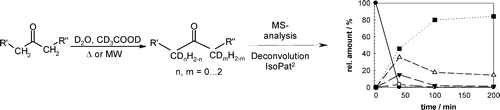
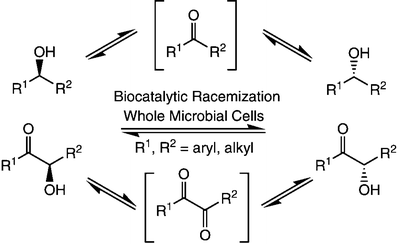
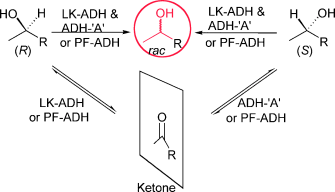
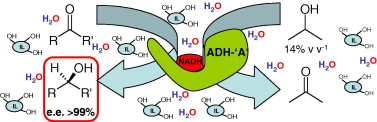
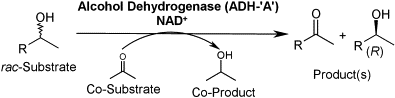
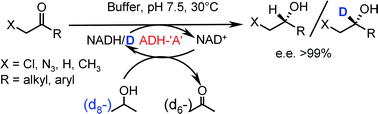
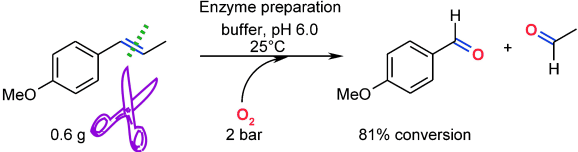
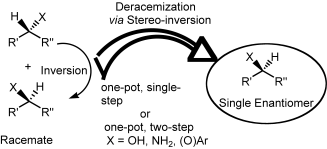
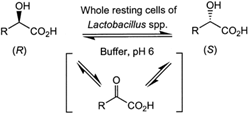
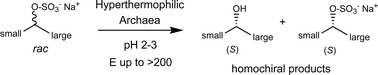
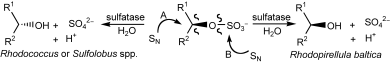
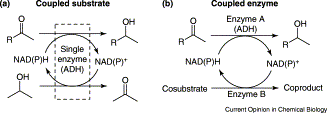
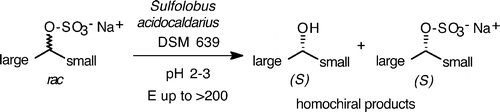
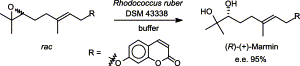
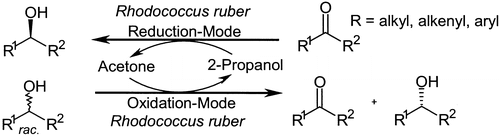
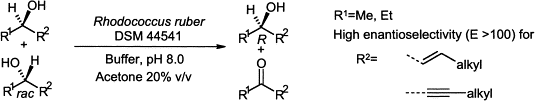
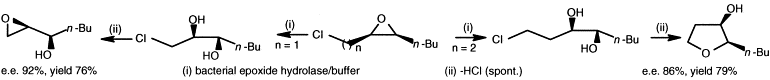
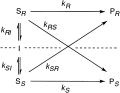
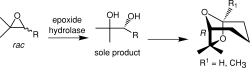
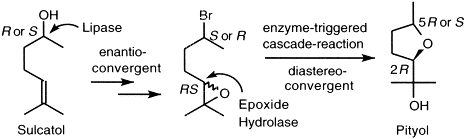
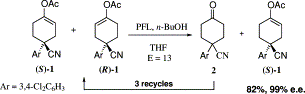
Photocatalysis and biocatalysis represent powerful efficient tools in synthetic chemistry. While, both have individually shown promising results, their integration remains challenging, particularly in continuous flow processes. This work presents a semicontinuous setup, combining photo- and biocatalysis in a multistep synthesis for the production of optically pure (S)-3,3,3-trifluoro-1-phenylpropan-1-ol. Initially, a photocatalytic trifluoromethylation of a methyl ketone in α-position in a self-made photoreactor was tested in flow, followed by enzymatic ketone reduction catalyzed by an alcohol dehydrogenase (variant of an ADH from Lactobacillus kefir). The study addresses the challenge of enzyme stability in aggressive solvents, developing a robust immobilization approach for the selected ADH with a PVA/PEG cryogel matrix. This strategy has been investigated in this work to ensure enzyme stability in THF, marking a notable advance in compatibility for continuous cascades. The separate process steps were finally combined in a semicontinuous flow system, achieving a space–time yield for the photocatalytic step of 39.8 g L−1 h−1 and of 1.12 g L−1 h−1 for the enzymatic step. The study signifies one of the first instances of combining photo- and biocatalysis in continuous cascades, offering an innovative approach to synthesizing chiral 3,3,3-trifluoro-1-phenylpropan-1-ol.







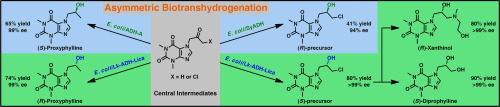
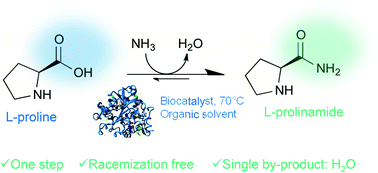
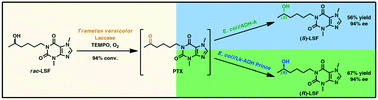
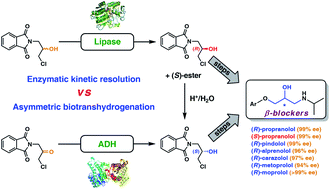



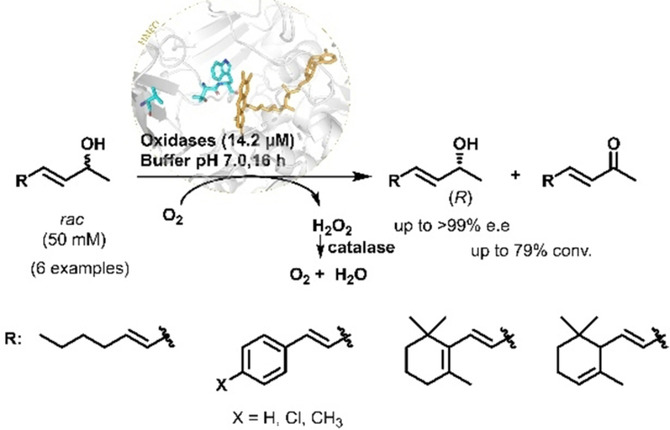
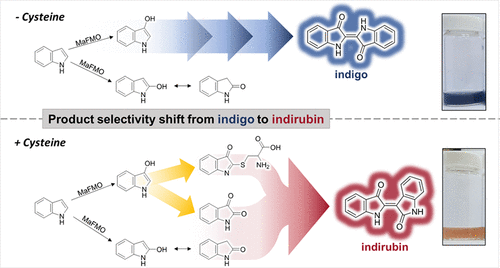
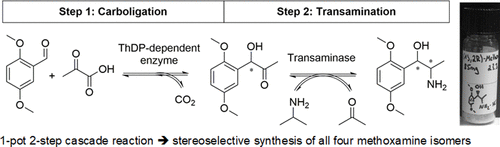

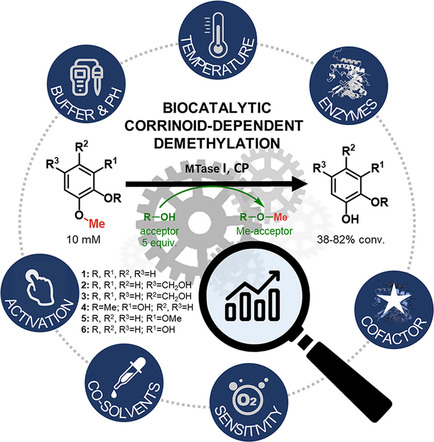

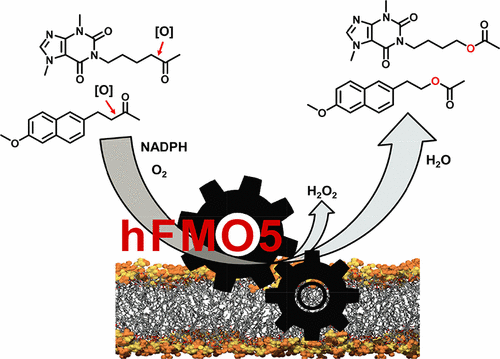
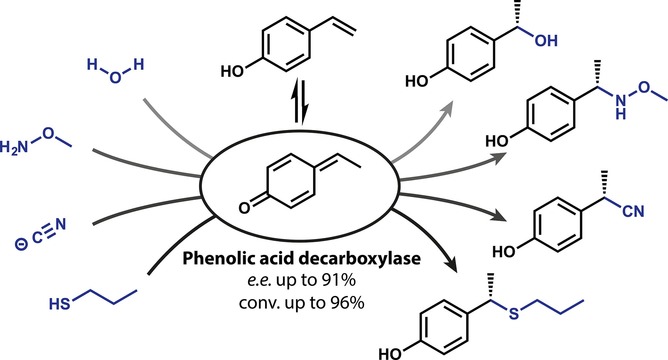
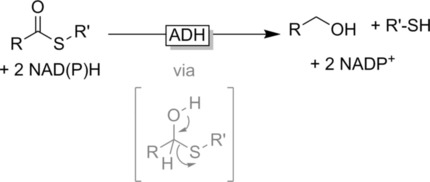
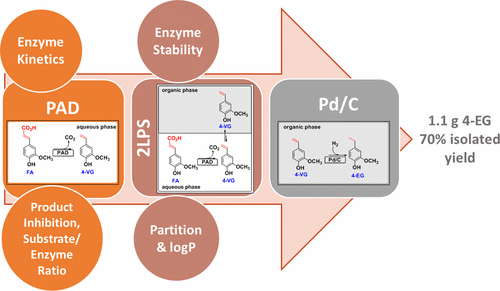
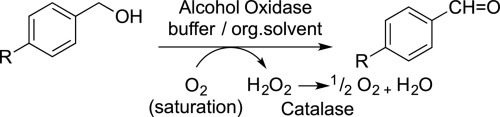
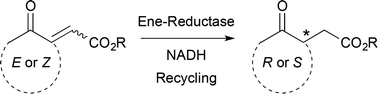
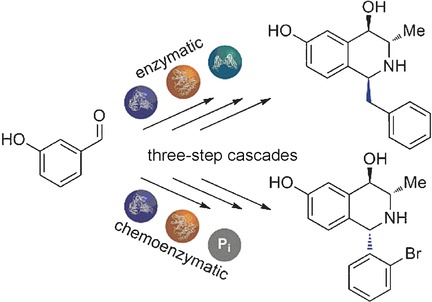

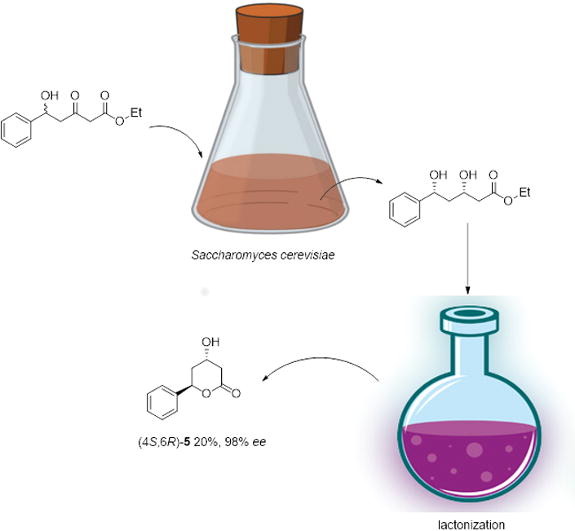
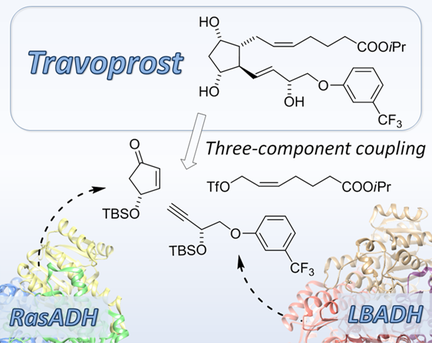
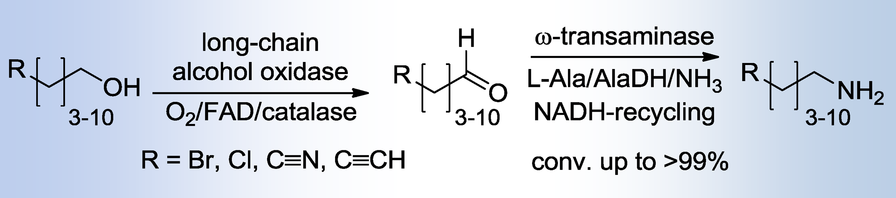
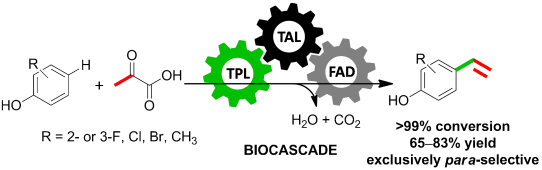

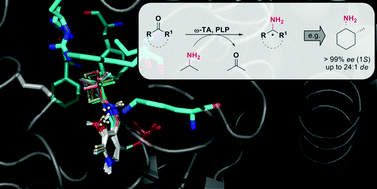
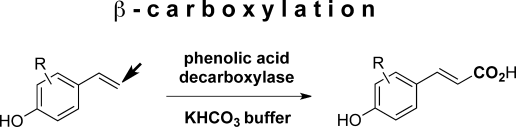
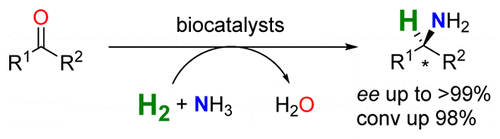

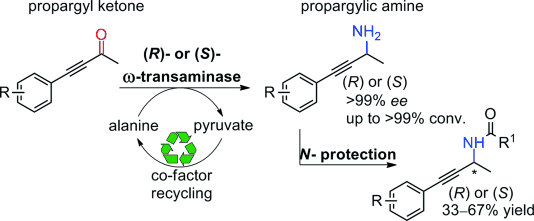
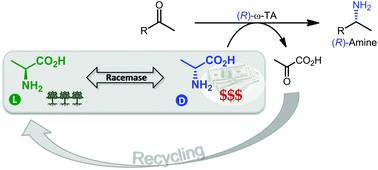



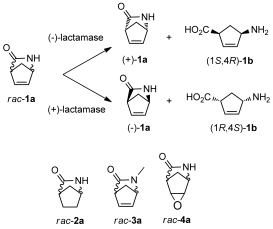
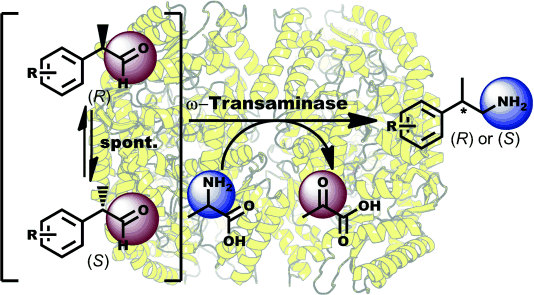
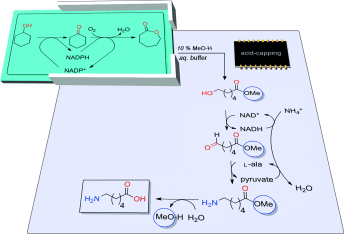
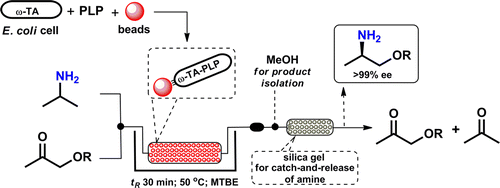





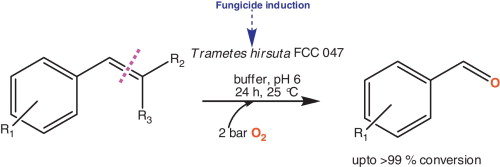
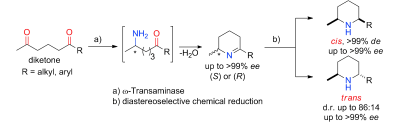

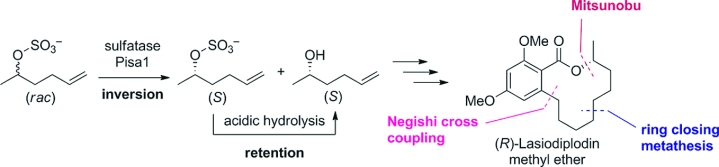

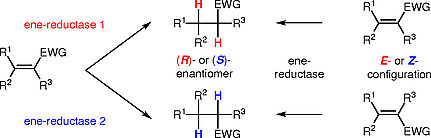
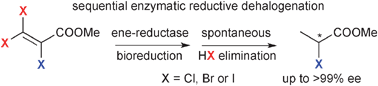
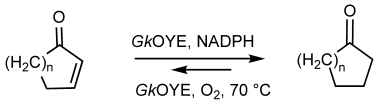


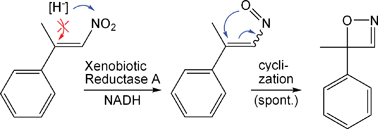
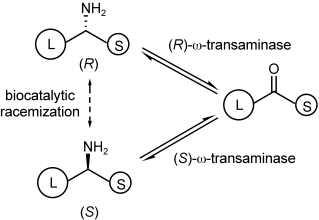
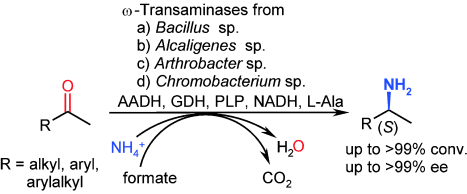
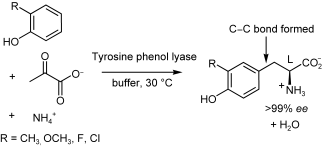
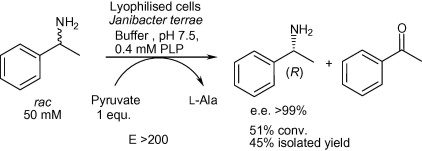
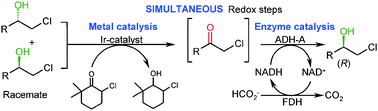
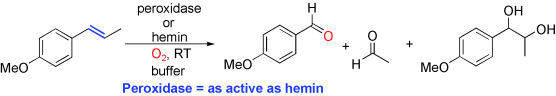
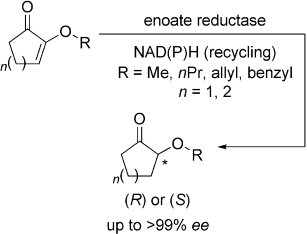
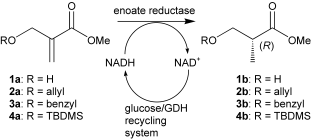
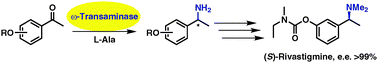

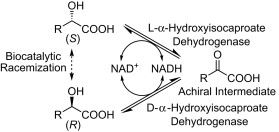
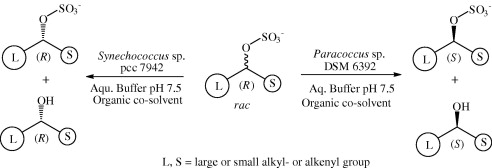
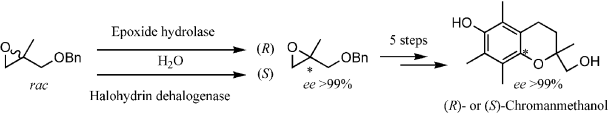
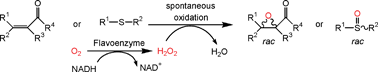









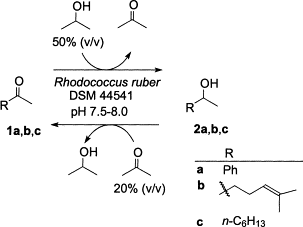

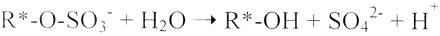
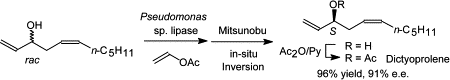
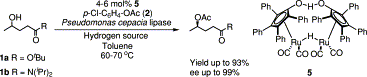
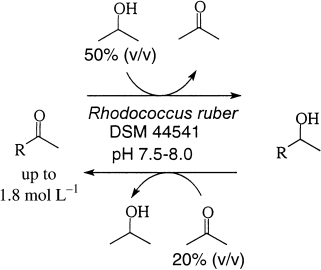
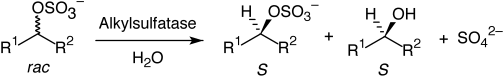
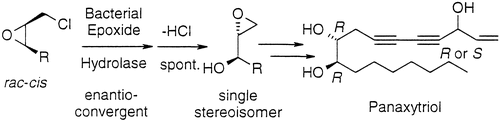
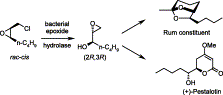
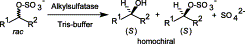
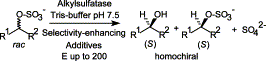

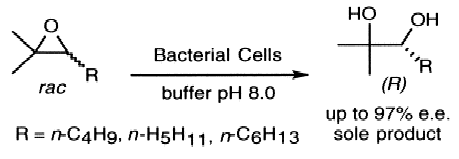

 .
.